For more information:
Email UsAnimal Care and Use Across Multiple Institutions
Obtaining and ensuring appropriate review, approval, and oversight of research involving animals can introduce significant challenges and delays for investigators and institutions, especially if review and approval must be sought from multiple Institutional Animal Care and Use Committees (IACUCs).
Institutions may choose to streamline IACUC review and oversight1 for collaborative, multisite research projects by establishing a formal written reliance agreement to address responsibilities for animal care, use, and ownership. However, setting up separate, protocol-specific agreements can be a time-consuming and resource-intensive process.
To address this problem, Harvard Catalyst’s Regulatory Foundations, Ethics, and Law program has developed an IACUC Agreement that may be used for IACUC reliance on a protocol-by-protocol basis. The agreement has been signed by 18 Harvard affiliates and others. Additional IACUC templates for use with non-signatories and vendors have been developed to assist institutions and researchers in off-site animal research arrangements.
Harvard Catalyst Agreement and Template Agreements for Animal Care and Use
The Reciprocal Institutional Agreement for Animal Care and Use (“IACUC Agreement”) supports the proper care and use of animals involved in multicenter studies among the signatory institutions. It is a formal umbrella agreement designed within a legal framework to reduce duplicative review of animal work.
Signatories
18 institutions currently participate in the IACUC Agreement:
| Beth Israel Deaconess Medical Center | Harvard University Faculty of Arts and Sciences (FAS) |
| Boston Children’s Hospital | Joslin Diabetes Center |
| Brigham and Women’s Hospital | Massachusetts Eye & Ear |
| Broad Institute | Massachusetts General Hospital |
| Dana-Farber Cancer Institute | Massachusetts Institute of Technology |
| Forsyth Institute | McLean Hospital |
| Harvard Medical School (HMS) | MGH Institute of Health Professions |
| Harvard T.H. Chan School of Public Health | Schepens Eye Research Institute |
| Harvard School of Dental Medicine | Spaulding Rehabilitation Hospital |
Examples of the IACUC Agreement in action
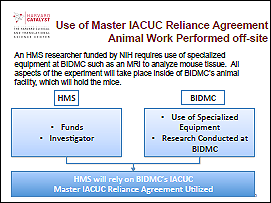
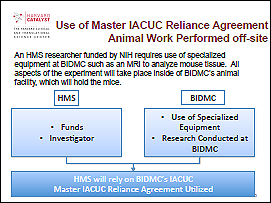
Example 1: Animal work performed off-site
In this example, the research involves animal activities in one location that is funded by a different institution. By utilizing the IACUC Agreement, one institution (e.g., the institution receiving the funds) may choose to defer review to the other (usually the one doing the animal work).
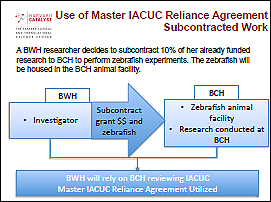
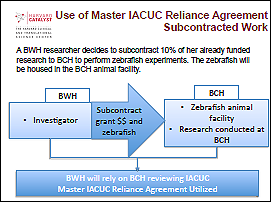
Example 2: Subcontracted work
In this example, the researcher is outsourcing the animal work to another signatory institution with the appropriate expertise and facilities for the animal model. By utilizing the IACUC Agreement, one institution may choose to perform the IACUC review and provides oversight for the research, so that administrative burden for the researcher as well as the institution is reduced.
When signatories join the IACUC Agreement they must agree to the following key elements:
- Eligibility Requirements
- Maintain an Animal Welfare Assurance and, if housing USDA-covered species, a USDA registration
- Maintain or aim to meet the standards required for accreditation from the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)
- Responsibilities
- Ownership: The institution in possession of the animals assumes ownership, unless otherwise agreed upon in advance. (See agreement for requirements during transport of animals)
- Congruence: While the institution receiving the funds is ultimately responsible for grant-protocol congruence, the parties may accept the institutional congruency review of the performance site, if agreed upon
- Protocol Review: The performance site is responsible for ensuring that animal care and use complies with PHS Policy, the Animal Welfare Act, the Guide for Care and Use, and other applicable laws, statutes, and guidance
- An appropriate institutional representative involved in a given collaboration may choose to:
- Attend the IACUC meeting at another institution where the protocol will be reviewed
- Visit the space within which animals are housed or used
- Request minutes or semi-annual reports
- Include the site in a post-approval monitoring audit/program
- Documentation, Notification, and Reporting
- The performance site is responsible for the regulatory requirements of animal care and use, such as maintaining all required documentation, reporting to federal, state, and local agencies as required, and providing this information to the relying site upon request (e.g., protocol documents or approvals) or as a rule (significant deficiencies, reports of non-compliance, USDA inspection reports)
- The institution receiving the grant is responsible for the financial regulatory requirements such as reporting to the funder any issues of non-compliance
- The performance site must inform the relying site(s) of a loss of OLAW assurance, USDA registration, or AAALAC accreditation.
- Each institution will submit its own annual reports to OLAW, USDA, etc
- An institution in receipt of a FOIA request forwards that request to the other institution participating in that specific research protocol
Example 1: Animal work performed off-site
In this example, the research involves animal activities in one location that is funded by a different institution. By utilizing the IACUC Agreement, one institution (e.g., the institution receiving the funds) may choose to defer review to the other (usually the one doing the animal work).
Example 2: Subcontracted work
In this example, the researcher is outsourcing the animal work to another signatory institution with the appropriate expertise and facilities for the animal model. By utilizing the IACUC Agreement, one institution may choose to perform the IACUC review and provides oversight for the research, so that administrative burden for the researcher as well as the institution is reduced.
You may use or modify the following templates with non-signatory institutions or vendors to speed the conduct of research involving animals while ensuring that all research is conducted ethically and with the appropriate oversight.
Template IACUC Agreement 1: Off-site Assurance
(Request by emailing regulatory@catalyst.harvard.edu)
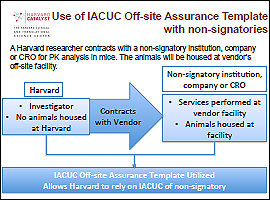
For use with institutions that are NOT signatories of the IACUC Agreement.
Example:
In this scenario, the researcher is contracting with a facility to perform animal activities on behalf of Harvard. As the vendor is not a signatory to the IACUC Agreement, the Offsite Assurance Template may be used to establish a written agreement for one institution to assume oversight of the animal work.
Template IACUC Agreement 2: Custom Antibodies
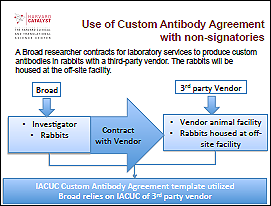
(Request by emailing regulatory@catalyst.harvard.edu)
For use when contracting with a company, vendor, or academic institution for production of antibodies specific to the research.
Example:
In this scenario, the researcher is contracting with a third-party vendor to perform custom antibody services on behalf of the Broad Institute of MIT and Harvard. As the vendor is not a signatory of the IACUC Agreement, the Custom Antibody Agreement Template can be used to establish a written agreement for oversight of the animal work.
Investigators or institutions that want to utilize the IACUC Agreement or inquire about use of the templates for a proposed or existing research project should contact their respective IACUC office.
For questions about who to contact, please email us at regulatory@catalyst.harvard.edu.
Join
Additional signatories may join the IACUC Agreement. Interested in joining? Please email regulatory@catalyst.harvard.edu for more information.
Contact
To share your ideas for IACUC agreements, templates, and forms, or for more information about the IACUC Agreement, please email regulatory@catalyst.harvard.edu.
To request any of the above documents be made accessible please email communications@catalyst.harvard.edu.