For more information:
Email UsCurriculum Development Process
The Education program uses Understanding By Design (UbD) as the selected development model to guide curriculum, assessment, and instruction planning. UbD focuses on teaching and assessing for learning by designing curriculum backwards, concentrating on the desired end results first. The process depicted below has been tailored for developing professional education materials such as ours.
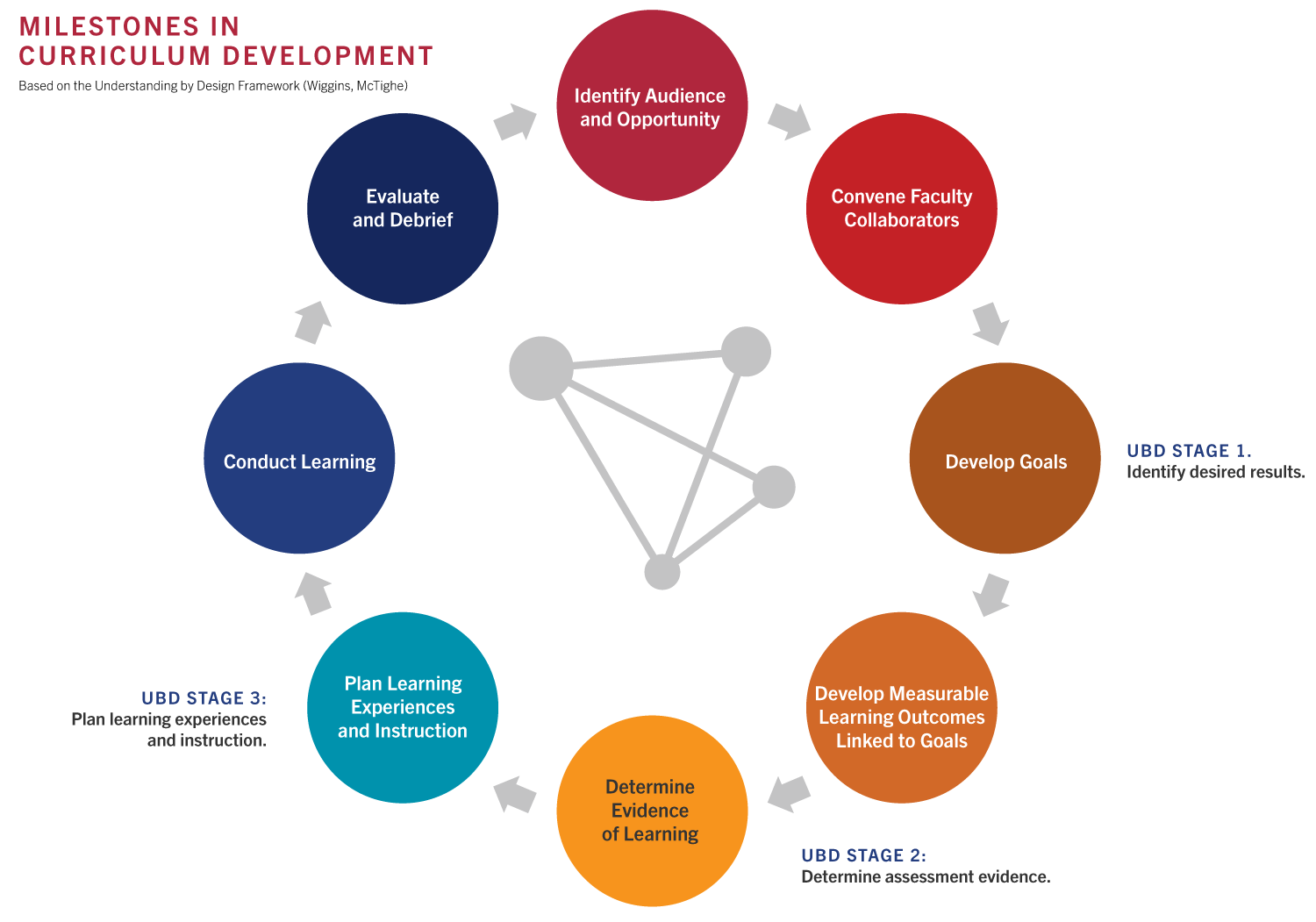
Guiding Competencies and Principles
Our team uses well-established competency frameworks to ensure all educational content trains c/t research professionals in the specific skills and characteristics required for career success. Frequently cited competency lists include those developed by the NIH/NCATS Center for Leading Innovation and Collaboration and the Joint Task Force for Clinical Trial Competency.
Additionally, all of our educational content aims to prepare c/t research professionals to respond to the imperative to increase diverse representation in research. To achieve this, we use as our foundation the six comprehensive principles from Achieving Diversity, Inclusion, and Equity in Clinical Research, an extensive guidance document published by the Multi-Regional Clinical Trials Center. These principles serve as a reference point throughout the three UbD phases, empowering content experts to identify and highlight how and where the principles show up in practice. Because the curriculum development process is iterative, these principles inform not only the development of new content, but also the revision and enhancement of existing content.
Understanding by Design (book supporting curriculum process)
Wiggins GP, McTighe J, Kiernan LJ. Understanding by Design. Alexandria, VA: Association for Supervision and Curriculum Development; 1998.
Our Conflict of Interest (COI) Policy
We partner with a diverse group of subject matter experts (including faculty, practitioners, and consultants), many of whom work and collaborate with external organizations, industries, and funding agencies. Some existing financial relationships with external entities may pose potential conflicts of interest. In order to ensure that commercial bias does not influence our educational content, our team invites subject matter experts to disclose relevant financial relationships with ineligible companies, and then implements mitigation steps throughout the curriculum development process as needed. For additional information about our COI policy, please contact us at education@catalyst.harvard.edu.

